Organometallic compounds have at least one carbon to metal bond, according to most definitions. This bond can be either a direct carbon to metal bond ( σ bond or sigma bond) or a metal complex bond ( π bond or pi bond). Compounds containing metal to hydrogen bonds as well as some compounds containing nonmetallic ( metalloid ) elements bonded to carbon are sometimes included in this class of compounds. Some common properties of organometallic compounds are relatively low melting points, insolubility in water, solubility in ether and related solvents, toxicity, oxidizability, and high reactivity.
An example of an organometallic compound of importance years ago is tetraethyllead (Et 4 4Pb) which is an antiknock agent for gasoline. It is presently banned from use in the United States.
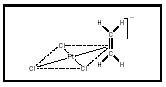
The first metal complex identified as an organometallic compound was a salt, K(C 2 H 4 )PtCl 3 , obtained from reaction of ethylene with platinum (II) chloride by William Zeise in 1825. It was not until much later (1951–1952) that the correct structure of Zeise's compound (see Figure 1) was reported in connection with the structure of a metallocene compound known as ferrocene (see Figure 2).
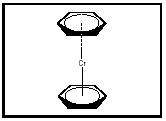
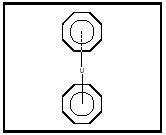
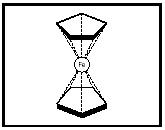 Preparation of ferrocene was reported at about the same time by two research groups, and a sandwich structure was proposed, based on ferrocene's physical properties (Kauffman, pp. 185–186). The sandwich structure was confirmed by x-ray diffraction studies. Since then, other metallocenes composed of other metals and other carbon ring molecules, such as dibenzenechromium (see Figure 3) and uranocene (see Figure 4), have been prepared.
Preparation of ferrocene was reported at about the same time by two research groups, and a sandwich structure was proposed, based on ferrocene's physical properties (Kauffman, pp. 185–186). The sandwich structure was confirmed by x-ray diffraction studies. Since then, other metallocenes composed of other metals and other carbon ring molecules, such as dibenzenechromium (see Figure 3) and uranocene (see Figure 4), have been prepared.
Possibly the first scientist to synthesize an organometallic compound was Edward Frankland, who prepared diethylzinc by reaction of ethyl iodide with zinc metal in 1849 (Thayer 1969b, pp. 764–765).
2 CH 3 CH 2 I + 2 Zn → CH 3 CH 2 ZnCH 2 CH 3 + ZnI 2
In organometallic compounds, most p-electrons of transition metals conform to an empirical rule called the 18-electron rule. This rule assumes that the metal atom accepts from its ligands the number of electrons needed in order for it to attain the electronic configuration of the next noble gas . It assumes that the valence shells of the metal atom will contain 18 electrons. Thus, the sum of the number of d electrons plus the number of electrons supplied by the ligands will be 18. Ferrocene, for example, has 6 d electrons from Fe(II), plus 2 × 6 electrons from the two 5-membered rings, for a total of 18. (There are exceptions to this rule, however.)
 Possibly the earliest biomedical application of an organometallic compound was the discovery, by Paul Ehrlich, of the organoarsenical Salvarsan, the first antisyphilitic agent. Salvarsan and other organoarsenicals are sometimes listed as organometallics even though arsenic is not a true metal. Vitamin B 12 is an organocobalt complex essential to the diet of human beings. Absence of or deficiency of B 12 in the diet (or a body's inability to absorb it) is the cause of pernicious anemia.
Possibly the earliest biomedical application of an organometallic compound was the discovery, by Paul Ehrlich, of the organoarsenical Salvarsan, the first antisyphilitic agent. Salvarsan and other organoarsenicals are sometimes listed as organometallics even though arsenic is not a true metal. Vitamin B 12 is an organocobalt complex essential to the diet of human beings. Absence of or deficiency of B 12 in the diet (or a body's inability to absorb it) is the cause of pernicious anemia.
An example of an organometallic compound of importance years ago is tetraethyllead (Et 4 4Pb) which is an antiknock agent for gasoline. It is presently banned from use in the United States.
The first metal complex identified as an organometallic compound was a salt, K(C 2 H 4 )PtCl 3 , obtained from reaction of ethylene with platinum (II) chloride by William Zeise in 1825. It was not until much later (1951–1952) that the correct structure of Zeise's compound (see Figure 1) was reported in connection with the structure of a metallocene compound known as ferrocene (see Figure 2).

Figure 1. Anion of Zeise's compound

Figure 2. Ferrocene
Possibly the first scientist to synthesize an organometallic compound was Edward Frankland, who prepared diethylzinc by reaction of ethyl iodide with zinc metal in 1849 (Thayer 1969b, pp. 764–765).
2 CH 3 CH 2 I + 2 Zn → CH 3 CH 2 ZnCH 2 CH 3 + ZnI 2
In organometallic compounds, most p-electrons of transition metals conform to an empirical rule called the 18-electron rule. This rule assumes that the metal atom accepts from its ligands the number of electrons needed in order for it to attain the electronic configuration of the next noble gas . It assumes that the valence shells of the metal atom will contain 18 electrons. Thus, the sum of the number of d electrons plus the number of electrons supplied by the ligands will be 18. Ferrocene, for example, has 6 d electrons from Fe(II), plus 2 × 6 electrons from the two 5-membered rings, for a total of 18. (There are exceptions to this rule, however.)

Figure 3. Dibenzenechromium
Use as Reagents or Catalysts
Organometallic compounds are very useful as catalysts or reagents in the synthesis of organic compounds, such as pharmaceutical products. One of the major advantages of organometallic compounds, as compared with organic or inorganic compounds, is their high reactivity. Reactions that cannot be carried out with the usual types of organic reagents can sometimes be easily carried out using one of a wide variety of available organometallics. A second advantage is the high reaction selectivity that is often achieved via the use of organometallic catalysts. For example, ordinary free-radical polymerization of ethylene yields a waxy low-density polyethylene, but use of a special organometallic catalyst produces a more ordered linear polyethylene with a higher density, a higher melting point , and a greater strength. A third advantage is that many in this wide range of compounds are stable, and many of these have found uses as medicinals and pesticides. A fourth advantage is the case of recovery of pure metals. Isolation of a pure sample of an organometallic compound containing a desired metal can be readily accomplished, and the pure metal can then be easily obtained from the compound. (This is generally done via preparation of a pure metal carbonyl, such as Fe[CO] 5 or Ni[CO] 4 , followed by thermal decomposition.) Other commonly used organometallic compounds are organolithium, organozinc, and organocuprates (sometimes called Gilman reagents). The name "ferrocene" was coined by one of Harvard University professor R. B. Woodward's postdoctoral students, Mark Whiting. The entire class of transitional metal dicyclopentadienyl compounds quickly became known as "metallocenes" and this has since been expanded for compounds [(H 5 ‒C 5 H 5 ) 2M] in general. G. Wilkinson and Woodward published their results on ferrocene in 1952.

Figure 4. Uranocene
No comments:
Post a Comment